1. Hydroxy FA (HFA) accumulated by castor, Physaria (formerly Lesquerella) and other species are found in lubricants, nylon, dyes, soaps, inks and adhesives. Industry consumes 1.1-1.3 billion lbs of HFA each year and demand is growing rapidly.
2. Conjugated FA (CFA), such as α-eleostearic acid (18:3, 9-cis, 11-trans, 13-trans) are synthesized by conjugase enzymes (CONJ) in Momordica charantia [52] and other species. They are used in drying oils and as petroleum additives to reduce VOC emissions from paints and other coatings.
3. Cyclopropane FA (CpFA) are synthesized from 18:1 by the addition of a methyl group across the double bond through action of cyclopropane synthase (CPS) found in cotton, Sterculia foetida, and other species. CpFA are prized for oxidative stability while retaining low viscosity. They therefore have wide applications as lubricants and biodiesel.
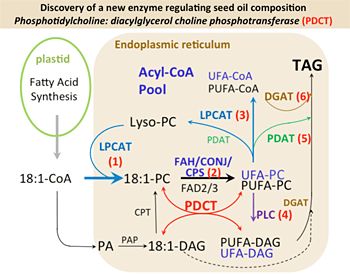
4. Our choice of these mFA underpins all of the advances we have made in this project to date. Most excitingly however, it is our comparative genomic analysis of these three oilseeds that led to the discovery that evolution has resulted in distinctly different paths for mFA metabolism through the network shown in Fig.1. Recent advances in genomics, sequencing, proteomics and bioinformatics technologies will now allow us to uncover the genomic and biochemical bases of these paths, as well as the other genetic determinants of successful, high-level accumulation of mFA in transgenic crop plants.